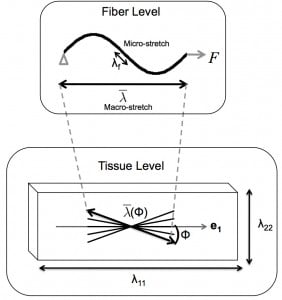
Soft collagenous tissues exhibit a highly organized structure, where stiff collagen fibrils embedded in a soft proteoglycan matrix are assembled into fibers and larger scale tissue-specific structures. The collagen structure can change in response to mechanical stimuli to produce tissue-level growth and remodeling of tissue-level properties. Growth and remodeling can occur as part of physiological processes, such as from regular exercise and pregnancy. However, disordered or unchecked growth and remodeling is a defining feature of many diseases, such as cardiac hypertrophy and glaucoma.
We seek to understand the micromechanisms through which mechanical stimuli direct collagen growth and remodeling. We hypothesize that the collagen structure is actively maintained or changed through concurrent processes of collagen deposition and degradation, and that the rates of collagen deposition and degradation can be altered by collagen mechanochemistry and by mechanosensitive collagen production and cellular contraction. Numerous experimental studies have shown that stretch can inhibit enzymatic degradation of collagen molecules, fibrils, and tissues. However, it is currently unknown if stretch has an analogous mechanochemical effect of enhancing collagen deposition. Mechanical stimulation can cause fibroblasts to contract and produce metalloproteinases (MMPs) and extracellular matrix (ECM) components. Mechanical loading can also induce fibroblasts to proliferate and differentiate into myofibroblasts, with increased contractility and ECM production. For example, myofibroblasts have been shown to develop in the mouse sclera within a week after a chronic increase in the intraocular pressure.
Our research aims to:
- Measure the effects of global mechanical and chemical stimuli on growth and remodeling
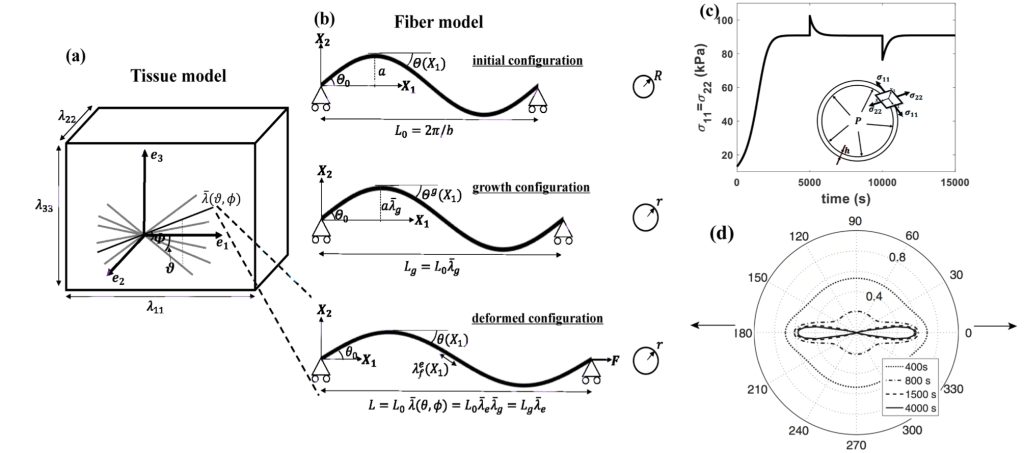
- Develop a micromechanical model for collagen growth and remodeling
- Investigate the effects of cell-scale perturbations on growth and remodeling.
Collaborators
Jeffrey Ruberti, Ph.D., Department of Bioengineering, Northeastern University
Daniel Reich, Ph.D. Department of Physics and Astronomy, Johns Hopkins University

