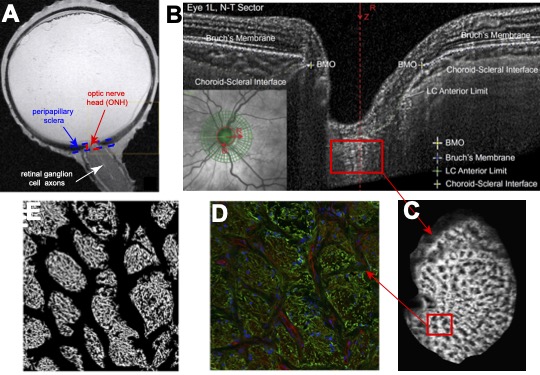
The optic nerve head (ONH) is a structure in the posterior eye-wall where the retinal ganglion cell (RGC) axons exit the eye (Figure 1A, reproduced from Norman et al. EER 90:277, 2010). The ONH consists of the prelaminar neural tissue (PLNT), the lamina cribrosa (LC), and the surrounding peripapillary sclera (PPS), choroid, retina, and retinal nerve fiber layer (RNFL) of the eye-wall (Fig. 1B). Astrocytes line the connective tissue beams of the LC and extend their processes into the pores to support the axon bundles (Fig. 1C-E). The goal of our work is to investigate the mechanical behavior of the tissues of the optic nerve head and how it remodels with glaucoma.
Structure and mechanical behavior of the LC
The LC is the connective tissue structure of the human optic nerve head. The LC serves to structurally support the glial cells, axons of the retinal ganglion cells, and nourishing capillaries of the optic nerve head.
Our research aims to:
- Measure the connective tissue structure and strain response to pressure of the human lamina cribrosa using multiphoton microscopy (second harmonic generation (SHG) and two photon fluorescent (TFP)), digital volume correlation (DVC), and morphological analysis.
- Investigate the effects of age, glaucoma diagnosis, and severity of axon damage
- Investigate mechanical interactions with the peripapillary sclera.
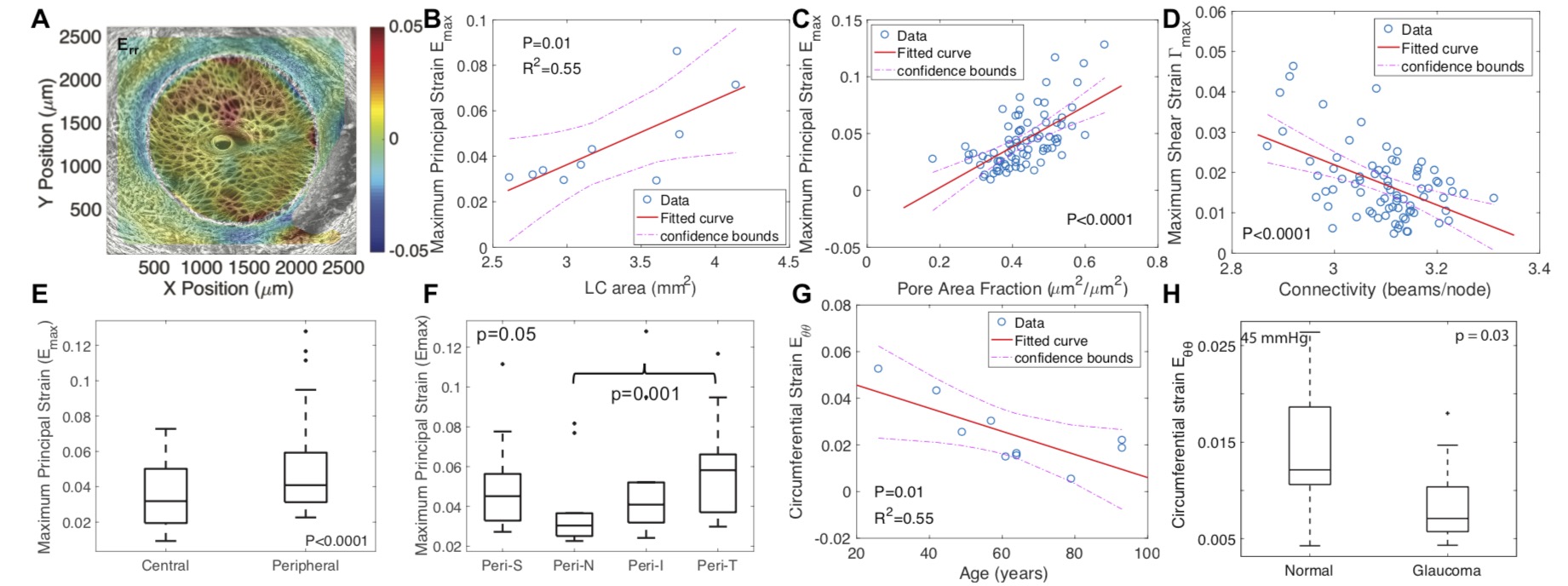
Figure 2 Analysis of SHG image volumes of the human LC by DVC for the strain response and by a custom morphological analysis program for connective tissue structure. (A) The thickness-averaged radial strain Err for inflation from 5-45 mmHg. (B) The average maximum principal strain Emax in the LC increased with LC area. (C) Emax was larger for regions with higher pore area fraction, while (D) the maximum shear strain was higher in regions with lower connectivity. (E) Emax was larger in the peripheral than central LC and (F) was smallest in the nasal quadrant; (G) Emax decreased with age. (H) The circumferential strain Eθθ was smaller in glaucoma eyes than age-matched normal eyes.
In vivo biomechanics of the ONH
The in vivo mechanical behavior of the human ONH are not well understood. Moreover the in vivo behavior may be different from the mechanical behavior measured in ex vivo tests because the ONH of explanted eyes have been removed from the physiological constrains and loading. In vivo measurements will also allow for longitudinal studies of the effects of glaucoma and glaucoma treatment on the remodeling of the ONH tissues.
Our research aims to:
- Measure the connective tissue structure and strain response to pressure of the human ONH in vivo using optical coherence tomography (OCT), digital volume correlation (DVC), and morphological analysis.
- Develop patient-specific models from OCT images to determine the material properties of the tissues of the ONH.
- Investigate the effects of structural damage and functional damage from glaucoma.
- Investigate remodeling of the ONH tissues with glaucoma and pressure-lowering treatment.
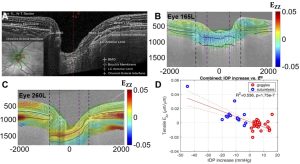
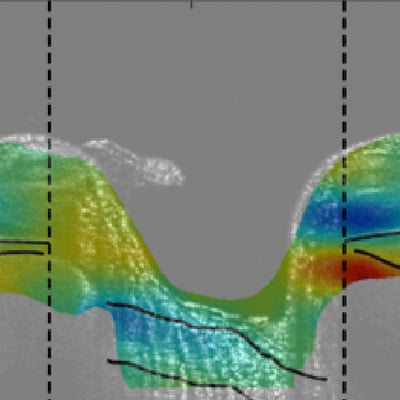
Figure 3: Analysis of radial OCT image volumes of the ONH by DVC to measure the full field strain response to an increase in the intraocular pressure (IOP) by wearing tight-fitting swim goggles without lens and to an IOP decrease following laster suturelysis. (A) OCT radial scan of the ONH after contrast enhancement. (B) Ezz is negative (compressive) for IOP increase from 21 to 23 mmHg. (C) Ezz is positive in response to a decrease in IOP from 31 to 21 mmHg. (D) A more compressive Ezz is significantly associated with a larger IOP increase.
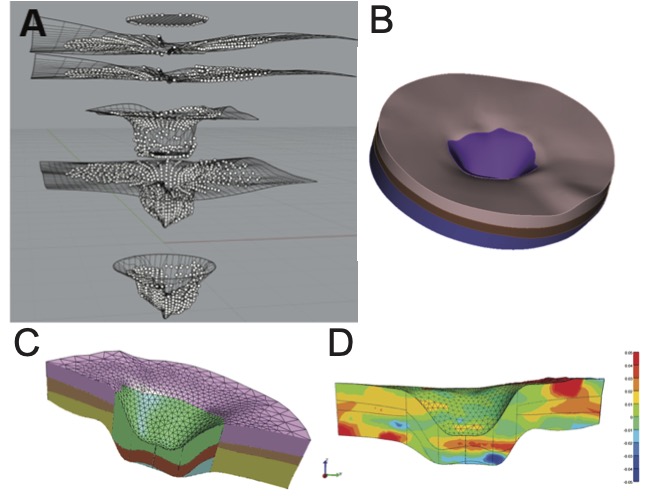
Figure 4: Creating a patient-specific model of the ONH from radial OCT scans.
Biomechanics and mechanobiology of mouse astrocytic lamina
The mouse ONH does not have a connective tissue lamina cribrosa. Instead, the optic axons are supported by a planar network of astrocytes called the astrocytic lamina (AL). Astrocytes are star-shape glial cells with long processes that encircle axonal bundles, forming a sieve-like network structure. The astrocyte provides physiological support as well as structural support to the axons, and thus presents a unique physical model to study the mechanobiology of ONH astrocytes in glaucoma.
Our research aims to:
- Measure the deformation response of the AL to pressure using, genetic mutants with fluorescent astrocytes, multi photon imaging of the AL, DVC analysis for full field deformation measurements, and morphological analysis.
- Develop specimen-specific finite element models and machine learning algorithms to study structure-strain and structure-stress relationship of the astrocyte processes and axonal compartments.
- Investigate mechanisms of IOP-induced astrocyte remodeling using chemical treatments.
- Investigate mechanical activation of astrocytes in 3D culture.
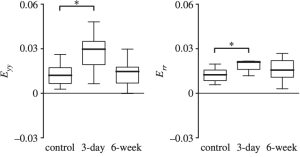
Figure 5: DVC analysis of TFP volume of the AL for the deformation response, showing (a) maximum intensity z-projection of the AL of a GPF-GFPA mouse, and contours of the circumferential and radial strain response to inflation from 10-30 mmHg. (b) The strain response becomes more compliant after 3 day of IOP elegant, but returns to baseline after 6 weeks of IOP elevation, suggesting that IOP elevation causes an short term remodeling of the AL and a return to homeostasis.
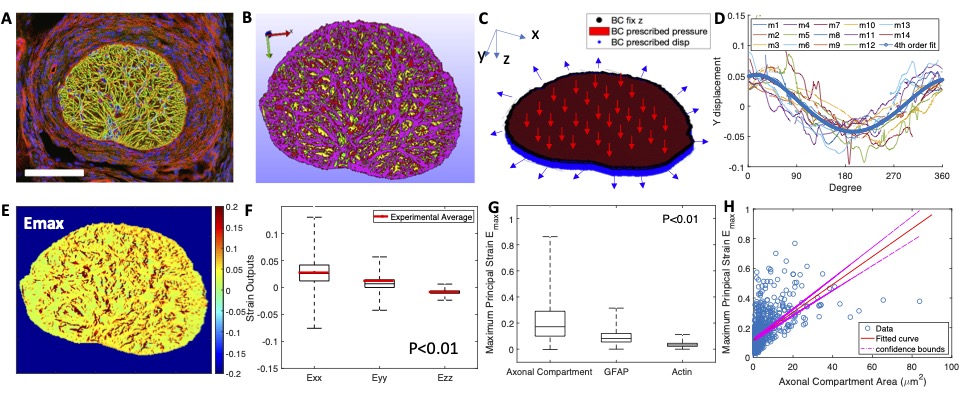
Figure 6: (A) Confocal fluorescent image off the mouse optic nerve stained for GFAP (green), actin (red), and nuclei (blue). (B) Finite element model with green fro GFAP, red for actin, yellow for axonal compartments, and purple for regions with overlapping GFAP and actin. (C) boundary displacements were determined from experiments (D). (E) Contour of the maximum principal strain Emax. (F) The average strain in model compares will with experiments. (G) Emax in the axonal compartments are much larger than in the astrocyte processes. (H) Larger axonal compartments experienced larger strains.
Collaborators
- Harry A. Quigley, MD – A. Edward Maumenee Professor of Ophthalmology, Director, Glaucoma Center of Excellence, Johns Hopkins Medical School